Heart Failure, Prostaglandins, and DNA Repair
THE LISTER AND PETERS PRIZES IN RESEARCH
 Barry Rubin
|
Barry Rubin, Medical Director
of the Peter Munk Cardiac
Centre, is also Head of the
Division of Vascular Surgery
at University Health Network.
He received his MD degree
from McGill University and his
General and Vascular training
at the University of Toronto,
completing a PhD in experimental
medicine in the Surgeon
Scientist Program. The only Wylie Scholar in Academic
Vascular Surgery from outside the United States, he
has been funded continuously by CIHR for 14 years.
He and his wife, Penny have 3 children (See Surgical Spotlight Winter 2008).
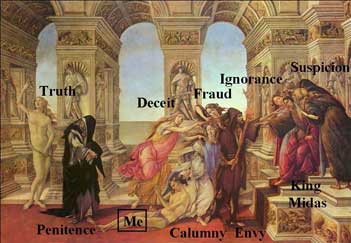
He opened with a fascinating discussion of the Calumny
of Appelles, a satirical painting by Sandro Boticelli,
painted in 1494. The painting shows King Midas surrounded
by advisors, Suspicion and Ignorance. Calumny
(or slander), dragging an unidentified man by the hair
is approaching the King, led by Envy, and attended by
Deceit and Fraud. In the distance is Truth, being gazed
at menacingly by Penitence. Barry told us that at the
beginning of his research career, he felt like the young
man being dragged by the hair, in pursuit of truth but
surrounded by uncertainty.
He then noted that his success in research was attributable
in large part to his mentors, Paul Walker and Wayne
Johnston, and his longtime colleague, Tom Lindsay.
He then presented a challenging narrative about the
molecular regulation of cardiac myocyte growth by prostaglandins.
Prostaglandin E2 (PGE2) is critical in the
evolution of cardiac injury following myocardial infarction.
The final step in PGE2 biosynthesis is catalyzed
by an enzyme called mPGES-1. Barry showed that mice
which lack mPGES-1 have lower levels of PGE2 and
worse left ventricular function after coronary artery ligation.
Selectively deleting mPGES-1 in white blood cells,
through the use of chimeric mice, also led to worse left
systolic and diastolic function after myocardial infarction.
This is the first demonstration that an enzyme that
controls prostaglandin biosynthesis by white blood cells
can modulate the way the heart repairs itself after a heart
attack. This body of work has direct clinical implications,
as the millions of patients that take an inhibitor of
cyclo- oxygenase-2 will be candidates to take inhibitors
of mPGES-1, which are currently in clinical trials. Barry
closed by acknowledging his network of collaborators
in Stockholm, Frankfurt, Boston, Seattle and Toronto,
and dedicated his presentation to Drs. Helmut Schmidt
(Frankfurt) and Shafie Fazel (Toronto).
The George Armstrong Peter prize for 2010 was
awarded to Subodh Verma. Previous winners include
William Gallie, Frederick Banting and Charles Tator
among others. Subodh received his MSc and PhD
from the University of British Columbia and his MD
from Calgary. He trained in Cardiac Surgery at the
University of Toronto while maintaining an extremely
prolific research program. His research has produced
over 190 peer-reviewed articles and he is the Canada
Research Chair in Atherosclerosis. This year, Subodh
was presented with the Howard Morgan Award from
the International Academy of Cardiovascular Sciences,
listed as one of Canada's Top 40 under 40 (http://www.theglobeandmail.com/report-on-business/managing/top-40-under-40-2009/subodh-verma-39-ontario/article1591369/)
and simultaneously placed on the India
Abroad Power List. His commitment to nurturing the
next generation is evidenced by the 2010 Silver Shovel
Award (excellence in overall clinical teaching as voted
by the University of Toronto medical students), his fervent
efforts to bring to life the St Michael's Li Ka Shing
Knowledge Institute-King Saud University collaborative
partnership and the recent achievements of his trainees
at the annual conferences of the American Association
for Thoracic Surgery (2010 C. Walton Lillehei Resident
Forum winner - Bobby Yanagawa (Cardiac Surgery resident)),
the American Heart Association (2010 Best Basic
Cardiovascular Sciences Presentation winner - Young
Kim (Cardiology resident), 2009 Vivien Thomas Young
Investigator Award winner - Krishna Singh (Postdoctoral
Fellow)) and the American College of Cardiology 2009
Young Investigator Award finalist - Praphulla Shukla
(Postdoctoral Fellow)).
Subodh thanked Richard Wiesel, Ren-Ke Li, Tirone
David, and David Latter for affording him the opportunity
to pursue his academic interests throughout his
Cardiac Surgery residency. He also expressed his gratitude
to his surgeon partners at St Michael's and Surgeonin-
Chief, Ori Rotstein, who collectively made it possible
for him to balance a productive research program with
the demands of an active clinical practice.
|
Subodh presented recent findings from his laboratory
linking the breast cancer gene BRCA1 to cardiovascular
disease which has culminated in the successful filing of
a U.S. provisional patent. In brief, BRCA1 is a genomewide
gatekeeper of DNA repair that has been widely
associated with breast, ovarian, and pancreatic cancer.
Subodh's "outside-the-box" line of investigation was
conceived after Bill Stanford (IBBME) described to him
the high incidence of premature heart failure-associated
deaths in a colony of Nbr1 (neighbor of BRCA1 gene)
mouse mutants.

Subodh Verma with his son, Raj Subhash and his daughter, Meena
Measurements of BRCA1 in the heart were initiated
and found to be very low in the naïve state, but there
were dramatic increases after induction of myocardial
infarction (MI). To definitively identify a potential role
for BRCA1 in cardiac physiology/pathology, Subodh
generated mice that had one or both copies of the
BRCA1 allele specifically deleted in cardiomyocytes.
Compared to the control mice, these animals exhibited
a higher incidence of ventricular rupture, impaired ventricular
function, extensive wall thinning and greater
mortality post-MI. Noteworthy, these are features
reminiscent of a human ischemic cardiomyopathic
phenotype. From a mechanistic standpoint, Subodh's
team went on to discover that the adverse cardiac
phenotype observed is p53-dependent and involves
in part elevated apoptosis and reduced repair of DNA
double-strand breaks. That the same unfavourable
cardiac pathology was documented in cardiomyocyte
- specific BRCA1 knockout mice following treatment
with the cardiotoxic anthracyclin doxorubicin lends
credence to the robustness of the notion that BRCA1
is cardioprotective. Work on three models of human
cardiac ischemia - atrial biopsies obtained before and
after initiation of cardiopulmonary bypass and aortic
cross clamping, ventricular samples from patients with
normal coronary arteries undergoing valvular surgery
and from those having coronary artery bypass graft
surgeries, and human fetal cardiomyocytes subjected to
(non-)ischemic conditions - has revealed that BRCA1
levels are significantly higher in the ischemic groups
thereby cementing the clinical relevance and translational
potential of this work. Further support stems
from recently collected results indicating that derangements
in BRCA1 expression and/or bioavailability may
resultantly alter substrate metabolism ensuing in an
energy starved heart and predisposition to ischemic and
non-ischemic heart failure.
The developing picture of the role of BRCA1 in the
heart is in brief: acute coronary syndrome causes cardiac
ischemia which leads to impaired repair of DNA
double-strand breaks and increased apoptosis which
can result in late heart failure and potentially cardiac
death. Accordingly, the clinical implications are that
BRCA1 mutation carriers and their families may be
at a previously unrecognized risk of heart failure. This
is especially thought-provoking since BRCA1 deficiency
has recently been associated with a significantly
increased risk of non-cancer related death via unidentified
mechanisms.
Three arms of research within Subodh's team have subsequently
sprouted the idea that BRCA1 and BRCA2 play an important role in other chronic diseases. With
regard to endothelial health, in vitro findings support
a role for BRCA1 in inhibiting endothelial apoptosis
and in improving endothelial function. Evidence from
human atherosclerotic samples show markedly attenuated
BRCA1 levels in plaque areas and gain-of-function
studies suggest that BRCA1-based cell or gene therapy
may represent a novel treatment approach for diseases
characterized by endothelial dysfunction, such as atherosclerosis.
Led by Hwee Teoh, PhD, an Associate Research Scientist, the group has also accumulated
extensive data demonstrating that BRCA1 gene therapy
not only retards experimental sepsis-associated multiorgan
dysfunction but importantly also limits postsepsis
mortality. Preliminary results pivoting around
BRCA2 have started to surface and promise to open yet
another avenue of investigation into the relevance of
oncogenes in cardiovascular medicine.
Subodh has recently initiated discussions with oncologists
at the H. Lee Moffitt Cancer Center in Florida to
spearhead a clinical study aimed at prospectively evaluating
cardiovascular risks in patients with BRCA1/2 mutations.
This dynamic team together with Steven Narod,
the Canada Research Chair in Breast Cancer, is also
looking into the potential of retrospectively assessing
cardiovascular risks and incidents in existing and ongoing
BRCA1/2 patient registries.
When not focusing on his patients and research, Subodh
enjoys and guards his down time shared with his two
children and who cannot wait for dog sledding season
to arrive.
M.M. with notes from Barry Rubin and Subodh Verma
|